 The French physicists Pierre and
Marie Curie
performed much of the ground breaking research into radioactivity.
After several years of study,
scientists identified several
distinct types of particles resulting from radioactive processes
(radiation).
The three distinct types of radiation were
named after the first three letters of the Greek alphabet:
The French physicists Pierre and
Marie Curie
performed much of the ground breaking research into radioactivity.
After several years of study,
scientists identified several
distinct types of particles resulting from radioactive processes
(radiation).
The three distinct types of radiation were
named after the first three letters of the Greek alphabet:
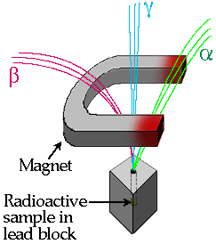
Alpha particles are helium nuclei (2 p, 2 n):
![]()
Beta particles are speedy electrons:
![]()
Gamma radiation is a stream of photons:
![]()
"X rays," "visible light," "radio waves," etc., are all photons at different energies. Gamma radiation refers to high-energy photons.
Alpha particles can be stopped by a sheet of paper, beta particles by aluminum, and gamma radiation by a block of lead. Since gamma radiation can penetrate very far into a material and has the ability to disrupt chemical bonds, it is gamma radiation that poses the most danger when working with radioactive materials (sadly, it took scientists many years to realize the perils of radioactivity....)