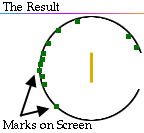
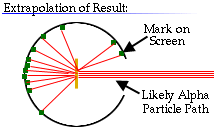


Rutherford reasoned that most of the alpha particles had easily passed
through the outer part of the atom, but that a few alpha particles must have
bounced off something inside the atom that was small, dense, and positively
charged.
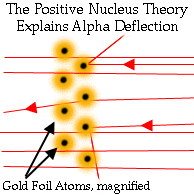 Although this disproved his hypothesis that atoms were permeable
balls, it provided him with a new hypothesis that atoms have
nuclei. This new model explained his results and was a keystone in the
development of modern atomic theory.
Although this disproved his hypothesis that atoms were permeable
balls, it provided him with a new hypothesis that atoms have
nuclei. This new model explained his results and was a keystone in the
development of modern atomic theory.